In more exciting research news for Genetic Cures for Kids (GC4K), the brain organoids that have been grown at AIBN from SPG56 patient stem cells have been dosed with the AAV9 viral gene therapy: the same one-dose therapy we are developing to treat patients with SPG56.
At the end of September Dr Hannah Leeson received the AAV9 vectors from our research team led by Dr Neil Hackett in the USA, and she treated the “mini brains” with a control vector expressing green fluorescent protein to make sure the vector goes into the brain organoids.
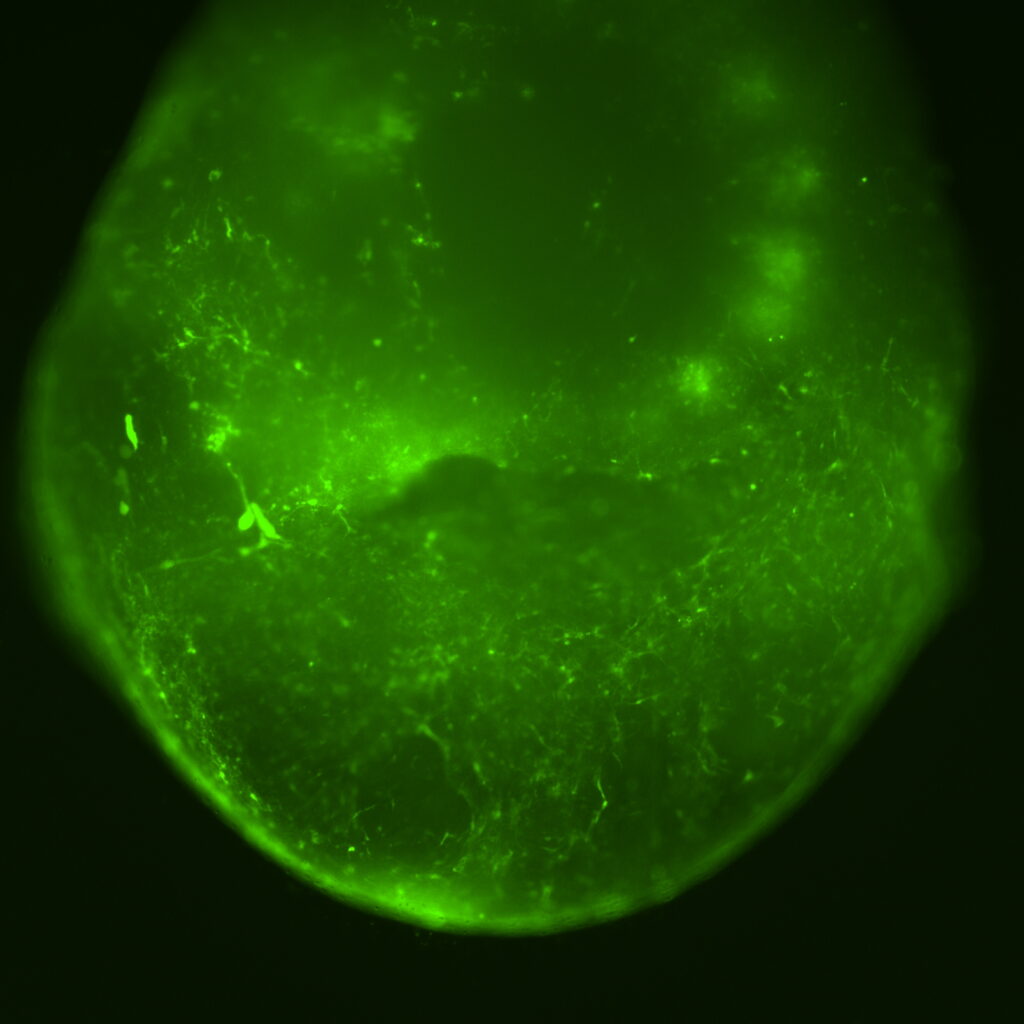
As expected, and hoped, the vector successfully entered the cells that make up the mini brains, taking with it the green fluorescent protein, which is now being expressed through the cells of the brain organoid! These organoids have been glowing green for 3 weeks now and are still going strong, promising news for the longevity of gene therapy – the longer the protein is expressed, the less often we need to treat with the vector. These beautiful glowing green mini-brains show that the method of gene therapy works, and Dr Leeson and Prof Ernst Wolvetang have now begun treating additional mini brains with the real deal: the AAV9 vector carrying the crucial CYP2U1 gene.
| What’s a CYP2U1 gene? It’s one of the genes that that Tallulah, and SPG56 children just like her, inherited from their parents. It’s also the gene that, due to its variant (a little glitch on the DNA strand) caused the cells to stop functioning as they should, and triggered the rare genetic disease SPG56. Using AAV9 gene therapy, doctors can replace the non-functional CYP2U1 gene with a synthetically designed and fully functioning CYP2U1 gene in an SPG56 patient’s Central Nervous System. That healthy CYP2U1 gene will then be expressed within the brain cells, (as the green fluorescent protein has) and return the functions needed to overcome SPG56. |
So, what happens now?
Our researchers observe and identify how the mini-brains heal when given the CYP2U1 gene via AAV9 gene therapy. Once we have a Proof of Concept that shows effective change, we take our research to the Therapeutic Goods Administration and put forward an Investigational New Drug application (IND). If approved, GC4K will then sign a contract with a vector manufacturer to build the life-changing vectors to test the safety of the gene therapy in order to make it to clinical trial.
2023 looks very hopeful for the SPG56 research program. But the research ahead is expensive: ensuring a novel therapy is safe requires many expert researchers, clinicians, and very high-tech labs. We are far off meeting our budget despite the incredible groundswell of support from the ‘Our Moon’s Mission’ Army. If you share our story with your networks, we increase our chances to find the philanthropic support we need to get to clinical trial.
It’s important to remember that this research will not only help children living with SPG56 now and into the future; the methods we are bringing to clinical trial will build a replicable framework that could be used to treat some of the 7000 other rare diseases in the world (and 10% of the human race). From little things, big things grow.

Thanks for sharing this news. We will continue the journey and do our best to raise gunds