The SPG56 drug discovery project has researchers working quickly to find an FDA approved drug that could be repurposed to treat SPG56 patients- possibly alleviating symptoms, or slowing/stopping disease progression. FDA approved drugs are preferred targets as they are easier to translate to clinical trial, and could also prove more accessible to the geographically dispersed SPG56 population.
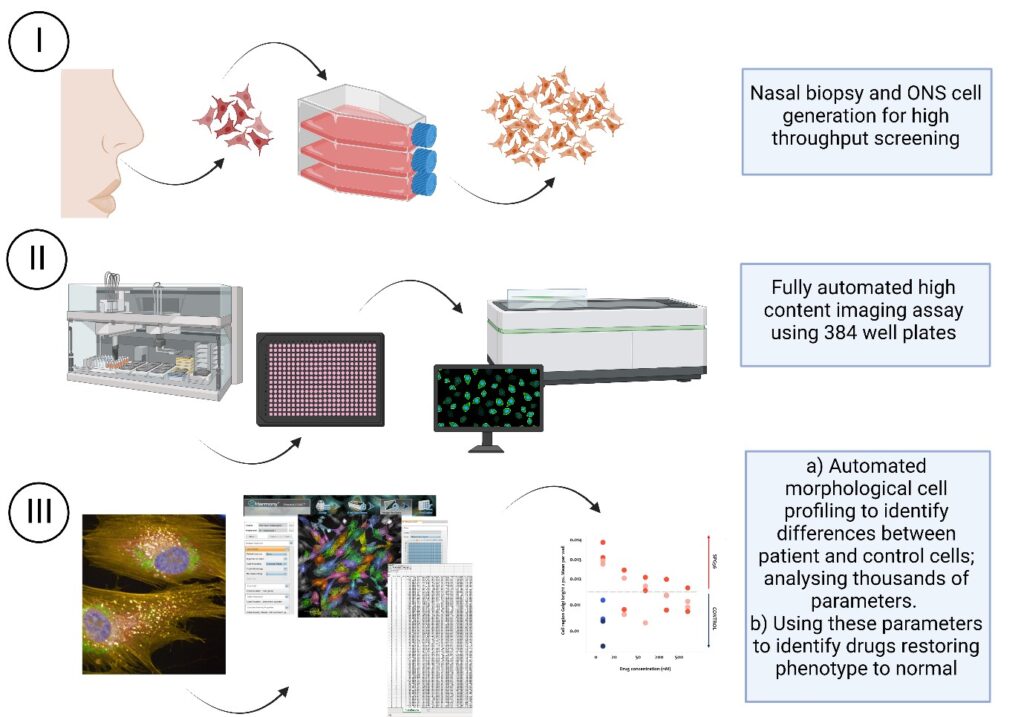
Genetic Cures for Kids is excited to share that the Griffith University (GU) team led by Professor Vicky Avery (Discovery Biology) have now optimised a high content imaging assay for undertaking high throughput screening using patient derived nasal olfactory stem cells. Our assay is in a fully automated, 384-well plate format capable of evaluating over 1200 cell features per cell/well.
| What’s high-throughput screening? High-throughput screening (HTS) is a process used for drug discovery that allows automated testing of large numbers of compounds or biological samples to find those which specifically impact on a particular target or cell/ organism. The Griffith University Discovery Biology team have extensive experience in assay development and HTS across many disease indications. The team uses multiple fully automated high content imaging systems and automated systems for plate processing. |
The successfully generated human olfactory neuroepithelial derived stem (hONS) cells were grown from nasal biopsies harvested in January and April 2022 from two children living with SPG56. This assay development has been an incredible achievement in such a short time.

Using SPG56 as the disease model, they are optimising a high throughput platform for drug discovery combining high-content imaging and machine learning to identify phenotypic profiles that differ between patient cells and controls. Importantly, this approach is transferrable across multiple rare genetic diseases as it does not rely on a specific target but rather differences in cell phenotype itself and will provide an alternative strategy to identify novel compounds for treatment of rare genetic diseases.
Importantly, this approach is tranferrable across multiple rare genetic diseases
Using HTS, the researchers’ objective is to identify compounds that reverse cell features of the patient samples, thus restore the phenotype and function to that of the normal cell. This project will validate phenotypic differences between patient cells and controls cells and identify small molecules that ameliorate or improve the SPG56 cell phenotype in a positive way.
The initial validation has generated preliminary data defining cell differences where restoration may be possible, which is being investigated further.
For SPG56, our team at Griffith University is currently finalising the data analysis workflow to be more efficient for analysing thousands of cell features obtained from screening many thousands of compounds against multiple patient derived cell lines. This is an exciting time as the team busily analyse and select the most appropriate compounds for initial testing, with a focus on FDA approved drugs currently used for other diseases.

This sophisticated approach is based on industry standards using state of the art equipment.
Please share our story with your networks and support our mission that will not only discover drugs to treat SPG56 but will also establish an approach for future drug discovery efforts for rare diseases across the world.
We are SO fortunate to have these skilled researchers in our own backyard, doing world-class research. Please help us continue this life-changing project!